QA-QC Protocols and Spectra
Introduction
This page contains spectra that are representative of the normal level of performance of this instrument. A brief description of the acquisition protocol is given for each set of measurements. More detailed information about the proper instrument set-up and operational parameters necessary to reproduce these measurements can be found in the appropriate training manual or application note.
NMR Specification Tests
Typically, the two parameters of concern in NMR are resolution/lineshape and sensitivity. This page contains information regarding the Production specifications and the Installed specifications for the Varian/Agilent MR400. It also contains a recent set of lineshape and sensitivity spectra for 1H and 13C for reference. Additional nuclei can be tested upon request. We ask that any time you have a question about lineshape or sensitivity, you first run one of the test protocols listed below in order to ensure that the issue is not related to your sample. Once the issue has been identified, additional troubleshooting of sensitivity and/or lineshape can proceed.
Resolution/Lineshape Test Protocol:
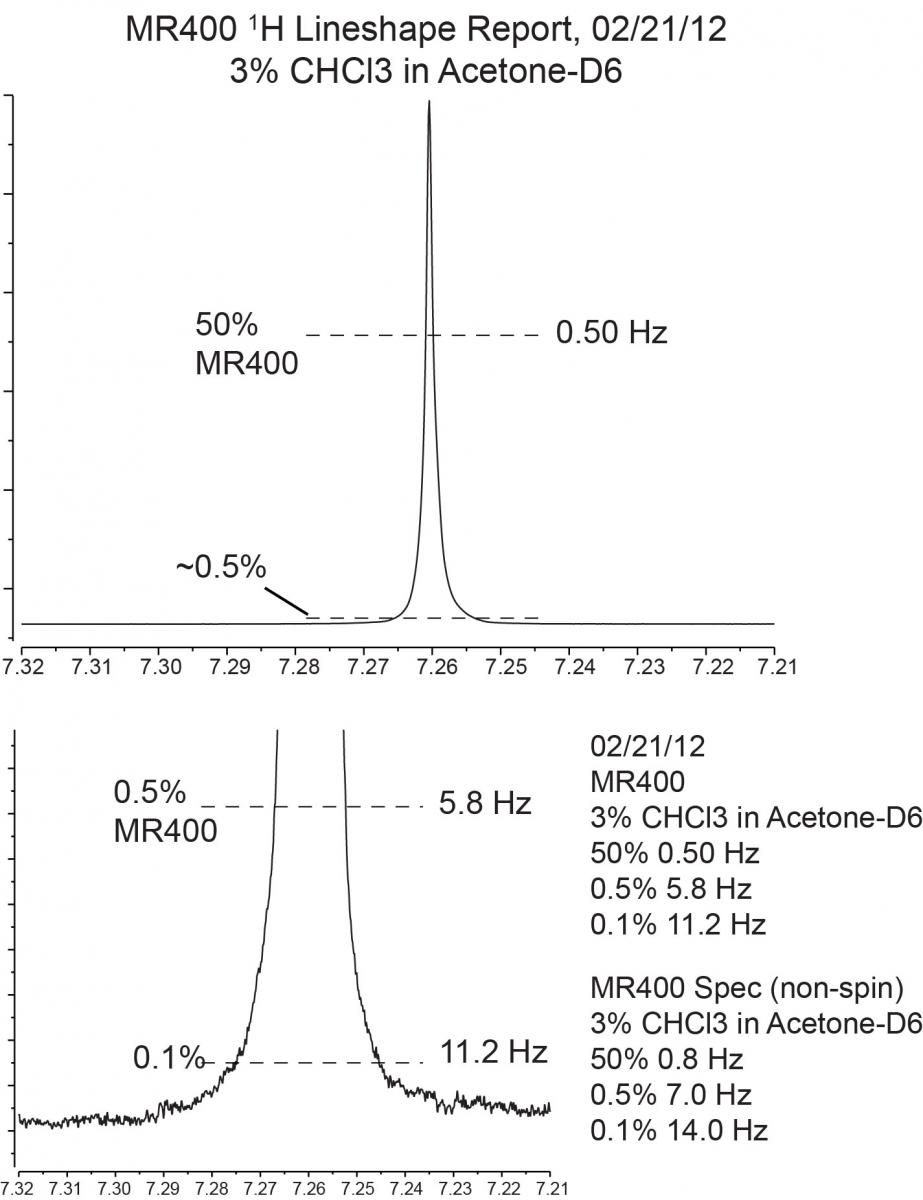
- Insert the 3% CHCl3 in acetone-d6 sample. The 1% sample is technically the sample used in the Agilent spec tests, but we can obtain the same lineshape using 3%. (In theory, 3% should give slightly broader lines because it is more concentrated.) For measuring 13C lineshape, use the 40% Dioxane in Benzene-D6 sample.
- Turn the spinning on if you are testing the resolution with spinning. If not, leave it off.
- Perform Find Z0, and Gradient Autoshim. If you are not spinning, you should optimize the X, Y, XZ, YZ shims. You may also wish to optimize Z5 and Z6 as they are not shimmed during Gradient Autoshim.
- Load a standard proton experiment. Change nt=1, np=80000. Type at? and the computer should return at=6.24. This parameter change is to ensure adequate acquisition time (at) in order to eliminate truncation and obtain the full resolution of the peak. Type su <enter> to read all of the changed parameters into the spectrometer.
- Type ga to start the acquisition (or click the Acquire button).
- When the experiment has finished acuiring, type wft<enter> and aph<enter> place the cursor on the chloroform peak and type res. The linewidth values for 50%/0.55%/0.11% will pop up on the top left corner of the spectrum.
- If the lineshape is significantly broader than spec (e.g. 50% > 1.0 Hz) send us an email containing the results of the lineshape test and we will re-shim the probe as soon as we are able. If the lineshape test meets spec but your particular sample has very broad lines, it is possible that something is wrong with your sample,or the instrument cannot shim your sample well, etc.
Typical MR 400 Lineshape: 50%/0.5%/0.1% = 0.8 Hz/7.0 Hz/14.0 Hz
Probe: 1H-19F/15N-31P 5mm PFG PZT OneNMR Probe, VT, 400 NB (54 mm)
Resolution and Lineshape (Production)
| Nucleus | Sample | 50% (in Hz) | 0.55% (in Hz) | 0.11% (in Hz) | Remarks |
|---|---|---|---|---|---|
| 1H | 1% Chloroform in Acetone-D6 | 0.45 | 5.0 | 10.0 | with rotation |
| 1H | 1% Chloroform in Acetone-D6 | 0.80 | 7.0 | 14.0 | without rotation |
| 13C | 40% Dioxane in Benzene-D6 | 0.15 | 1.5 | 3.0 | with rotation |
Resolution and Lineshape (Installed - October 2010)
| Nucleus | Sample | 50% (in Hz) | 0.55% (in Hz) | 0.11% (in Hz) | Remarks |
|---|---|---|---|---|---|
| 1H | 1% Chloroform in Acetone-D6 | 0.31 | 3.40 | 6.01 | with rotation |
| 1H | 1% Chloroform in Acetone-D6 | 0.43 | 6.66 | 10.26 | without rotation |
| 13C | 40% Dioxane in Benzene-D6 | 0.13 | 1.20 | 1.79 | with rotation |
Sensitivity (Production)
| Nucleus | Sample | Signal-to-Noise | Remarks |
|---|---|---|---|
| 1H | 0.1% Ethylbenzene (EB) in Chloroform-D | 480 | Wilmad 545-PP (Thin Wall), with sample rotation |
| 13C | 40% Dioxane in Benzene-D6 | 225 | Wilmad 545-PP (Thin Wall), with sample rotation |
| 13C | 10% Ethylbenzene (EB) in Chloroform-D | 175 | Wilmad 545-PP (Thin Wall), with sample rotation |
| 15N | 90% Formamide in DMSO-D6 | 20 | Wilmad 535-PP (Normal Wall), with sample rotation |
| 19F | 0.05 Trifluorotoluene in Chloroform-D | 550 | Wilmad 535-PP (Normal Wall), with sample rotation |
| 31P | 0.0485 M Triphenylphosphate in Acetone-D6 | 90 | Wilmad 535-PP (Normal Wall), with sample rotation |
Sensitivity (Installed - October 2010)
| Nucleus | Sample | Signal-to-Noise | Remarks |
|---|---|---|---|
| 1H | 0.1% Ethylbenzene (EB) in Chloroform-D | 514 | Wilmad 545-PP (Thin Wall), with sample rotation |
| 13C | 40% Dioxane in Benzene-D6 | 284 | Wilmad 545-PP (Thin Wall), with sample rotation |
| 13C | 10% Ethylbenzene (EB) in Chloroform-D | 194 | Wilmad 545-PP (Thin Wall), with sample rotation |
| 15N | 90% Formamide in DMSO-D6 | 22 | Wilmad 535-PP (Normal Wall), with sample rotation |
| 19F | 0.05 Trifluorotoluene in Chloroform-D | 671 | Wilmad 535-PP (Normal Wall), with sample rotation |
| 31P | 0.0485 M Triphenylphosphate in Acetone-D6 | 142 | Wilmad 535-PP (Normal Wall), with sample rotation |
Pulse Specificity (Production)
| Nucleus | Sample | 90° Pulse (in us) | Amp Used | RF Homogeneity |
|---|---|---|---|---|
| 1H | 1% 13C-Iodomethane | 7 | 50 |
810°/90°>70% 450°/90°>85% |
| 13C | 1% 13C-Iodomethane | 7 | 300 |
720°/0°>70% 360°/0°>85% |
| 15N | 90% Formamide | 14 | 300 | |
| 19F | 0.05% Trifluorotoluene in Chloroform-D | 8 | 50 | |
| 31P | 0.0485 M Triphenylphosphate in Acetone-d6 | 7 | 300 |
Pulse Specificity (Installed - October 2010)
| Nucleus | Sample | 90° Pulse (in us) | Amp Used | RF Homogeneity |
|---|---|---|---|---|
| 1H | 1% 13C-Iodomethane | 6.8 | 50 |
810°/90°= 78% 450°/90° = 87% |
| 13C | 1% 13C-Iodomethane | 5.4 | 300 |
720°/0° = 80% 360°/0° = 86% |
| 15N | 90% Formamide | 13.6 | 300 | |
| 19F | 0.05% Trifluorotoluene in Chloroform-D | 7.1 | 50 | |
| 31P | 0.0485 M Triphenylphosphate in Acetone-d6 | 6.4 | 300 |