Diffraction Basics
X-Ray Diffraction (XRD)
Q1. What is X-Ray diffraction and what are its applications?
Q2. Does XRD help determine the crystal structure and molecular formula?
Q3. What are the basic principles of XRD?
Q4. What is X-Ray crystallography?
Q5. What is a crystal structure?
Q1. What is X-ray Diffraction and what are its application in chemistry?
A1. The phenomena by which X-rays are reflected from the atoms in a crystalline solid is called diffraction. The diffracted X-rays generate a pattern that reveals structural orientation of each atom in a given compound.
X-ray diffraction is extensively used in chemistry for the characterization of organic and inorganic compounds that are made for pharmaceutical companies or making batteries of the cell phones.
XRD finds the geometry or shape of a molecule using X-rays. This technique is based on the elastic scattering of X-rays from structures that have long range order (crystalline solids). XRD technique is divided into two categories based on the morphology and size of sample:
1. If a crystal sample is large enough then it can be analyzed using X-ray Single Crystal diffraction, which solves for the complete structure ranging from simple inorganic solids to complex macromolecules. When we say “ crystals of large enough size” please note that they are still small enough for the normal eyes (most of the time like a speck of dirt!).
2. Does not form crystals large enough, then the sample is analyzed by using X-ray Powder diffraction (XRPD) technique. Powders of crystalline materials diffract X-rays. A beam of X-rays passing through a sample with randomly-oriented micro crystals produces a pattern of rings on a distant screen. XRPD provides less information than X-ray single crystal diffraction; however, it is much simple and faster. XRPD is useful for confirming the identity of a solid material and determining crystallinity and phase purity.
Q2. Does the XRD help in determining the crystal structure and molecular formula of a crystalline compound?
A1. XRD is an important method to characterize the structure of crystalline material. It can be used to determine either the lattice parameters, arrangement of individual atoms in a single crystal, or the phase anaylysis in case of polycrystalline materials and compunds. With the knowledge of XRD and crystallography, it is possible to determine the crystal structure and molecular formula of a crystalline compound.
Q3. What is the principle of XRD?
A3. XRD finds the geometry or shape of a molecule using X-rays. XRD techniques are based on the elastic scattering of X-rays from structures that have long range order. The X-rays get diffracted by a crystal because the wavelength of X-rays is similar to the inter-atomic spacing in the crystals.
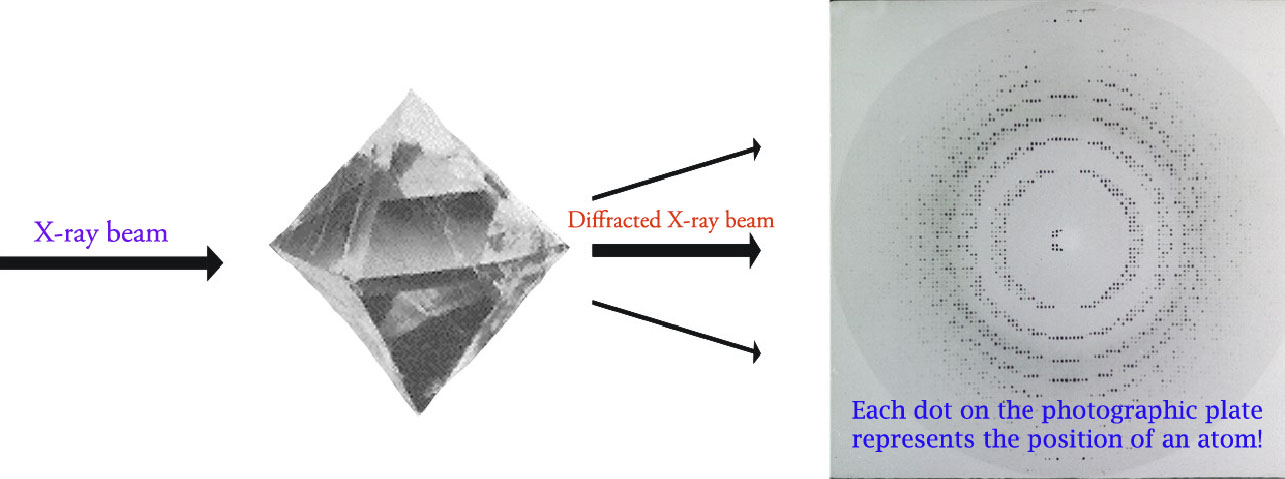
When the X-ray beam encounters the regular three-dimensional arrangments of atoms in a crystal, most of the X-rays will destructively interfere with each-other and cancel each-other out, but in some specific directions the X-ray beams interefere constructively and reinforce one another. It is these reinforced diffracted X-rays that produce the characteristic X-ray diffraction pattern that is used for crystal structure determination.
W.L. Bragg in the early 19th centuary showed that diffracted X-rays act as if they were 'reflected' from a family of planes within crystals. Later named after him, this Bragg's planes are the rows of atoms that make up the crystal structure as shown in the figure below.
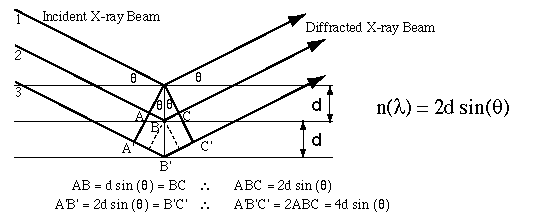
These reflections occur only under certain conditions which satisfy the equation:
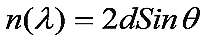
The above equation is also known as Bragg's equation. Here n is an intiger (1,2,3,...n), lambda is the wavelength, d is the distance between the atomic planes, and theta is the angle of incidence of the X-ray beams. An X-ray beam takes a longer (but parallel) path because if 'reflects' off an adjacent atomic plane. This path length difference must equal an integer value of the 1 of the incident X-ray beams for the constructive interference to occur such that a reinforced diffracted beam is produced.
Q4. What is X-ray Crystallography?
A4. X-ray crystallography is a method of determining the arragement of atoms within in a crystal, in which a beam of X-rays strikes a crystal and scatters into many different directions. From the angles and intensities of these scattered beams a three-dimensional picture of the density of electrons within the crystal can be produced. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their disorder and various other information. Since very many materials can form crystals - such as salts, metals, minerals, semiconductors, and various inorganic, organic and biological molecules. X-ray crystallography has been fundamental in the development of many scientific fields.
Q5. What is a Crystal Structure?
A5. A crystal structure is a unique arrangement of atoms/ molecules/ ions in a crystal. A crystal structure is composed of a motif, a set of atoms/ molecules/ ions arranged in a particular way, and a lattice. Motifs are located upon the points of a lattice, which is an array of points repeating periodically in three dimensions. The points can be thought of as forming identical tine boxes, called unit cells, that fill the space of the lattice. The lengths of the edges of a unit cell and the angles between them are called the lattice parameters.